CASE STUDY | IRCCS OSPEDALE SAN RAFFAELE · MILANO, ITALY
Excellent Outcomes With Stereotactic Body Radiotherapy in an Elderly Patient With Locally Progressive Immunotherapy-resistant Renal Cell Carcinoma
Authors:
Torrisi, Laura Giannini, Roberta Tummineri, Chiara Lucrezia Deantoni, Andrei Fodor, (IRCCS Ospedale San Raffaele – Milano, Italy)
Purpose:
Solution:
Study Design:
| Patient |
|
| Diagnosis | Diagnosed with clear cell RCC and pulmonary metastases |
| 2019 Treatment History |
|
| 2021 Treatment History |
|
| Treatment | Radixact SBRT, 36.25 Gy in five consecutive fractions. Daily ClearRT® kVCT imaging for patient alignment (see Figure 1) |
| Follow-up | Minimum of 5-6 months |
Treatment Details:
| Technology | CyberKnife® System with Synchrony® Fiducial Tracking™ with Respiratory Modelling |
| Fiducials | Four markers implanted; 3 in and 1 near the tumor |
| Planning Margins | 3 mm PTV margin; no CTV margin |
| Organs at Risk | Kidneys, bowel, spinal cord, liver (figure 1) |
| Prescription | 40 Gy in 5 consecutive fractions over one week (figure 2) |
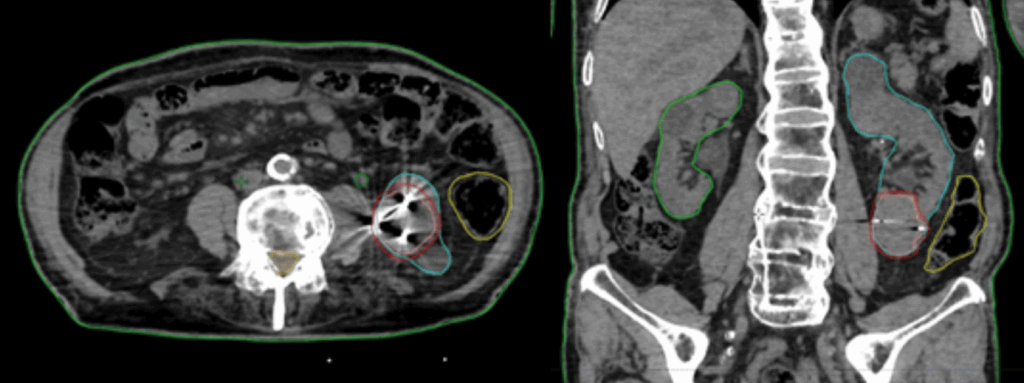
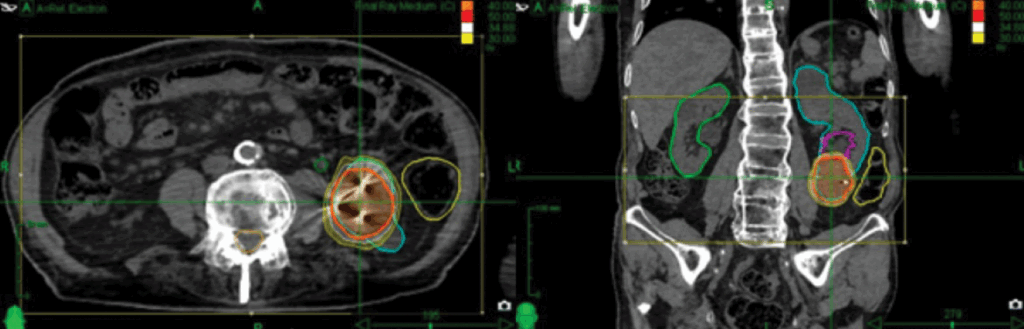
Outcomes:
| Toxicity | No acute grade ≥2 toxicity reported |
| Clinical Outcome |
|
| Renal Function | Renal function preserved; creatinine remained stable post-SBRT |
Conclusion:
“This case highlights the potential feasibility and safety of SBRT as a local treatment option for RCC in patients with mRCC. The durable disease control observed despite discontinuation of immunotherapy suggests that SBRT may have a valuable role in selected clinical scenarios.”
Torrisi M, Giannini L, Tummineri R, et al. (June 05, 2025) Excellent Outcomes With Stereotactic Body Radiotherapy in an Elderly Patient With Locally Progressive Immunotherapy-Resistant Renal Cell Carcinoma. Cureus 17(6): e85399. doi:10.7759/cureus.85399
Synchrony® is available in the US and EU markets. This feature may not be commercially available in all markets and is subject to regulatory clearance or approval. Kindly contact your regional Accuray representative or distributor to confirm the regulatory status.
Important Safety Information:
Most side effects of radiotherapy, including radiotherapy delivered with Accuray systems, are mild and temporary, often involving fatigue, nausea, and skin irritation. Side effects can be severe, however, leading to pain, alterations in normal body functions (for example, urinary or salivary function), deterioration of quality of life, permanent injury, and even death. Side effects can occur during or shortly after radiation treatment or in the months and years following radiation. The nature and severity of side effects depend on many factors, including the size and location of the treated tumor, the treatment technique (for example, the radiation dose), and the patient’s general medical condition, to name a few. For more details about the side effects of your radiation therapy, and to see if treatment with an Accuray product is right for you, ask your doctor. Accuray Incorporated as a medical device manufacturer cannot and does not recommend specific treatment approaches. Individual results may vary.
© 2025 Accuray Incorporated. All Rights Reserved. Accuray, the Accuray logo, and other trademarks are trademarks or registered trademarks of Accuray Incorporated and may not be used without permission. For more information on Accuray and its trademarks, please visit www.accuray.com/trademarks. MKTCKCSCC280925#10